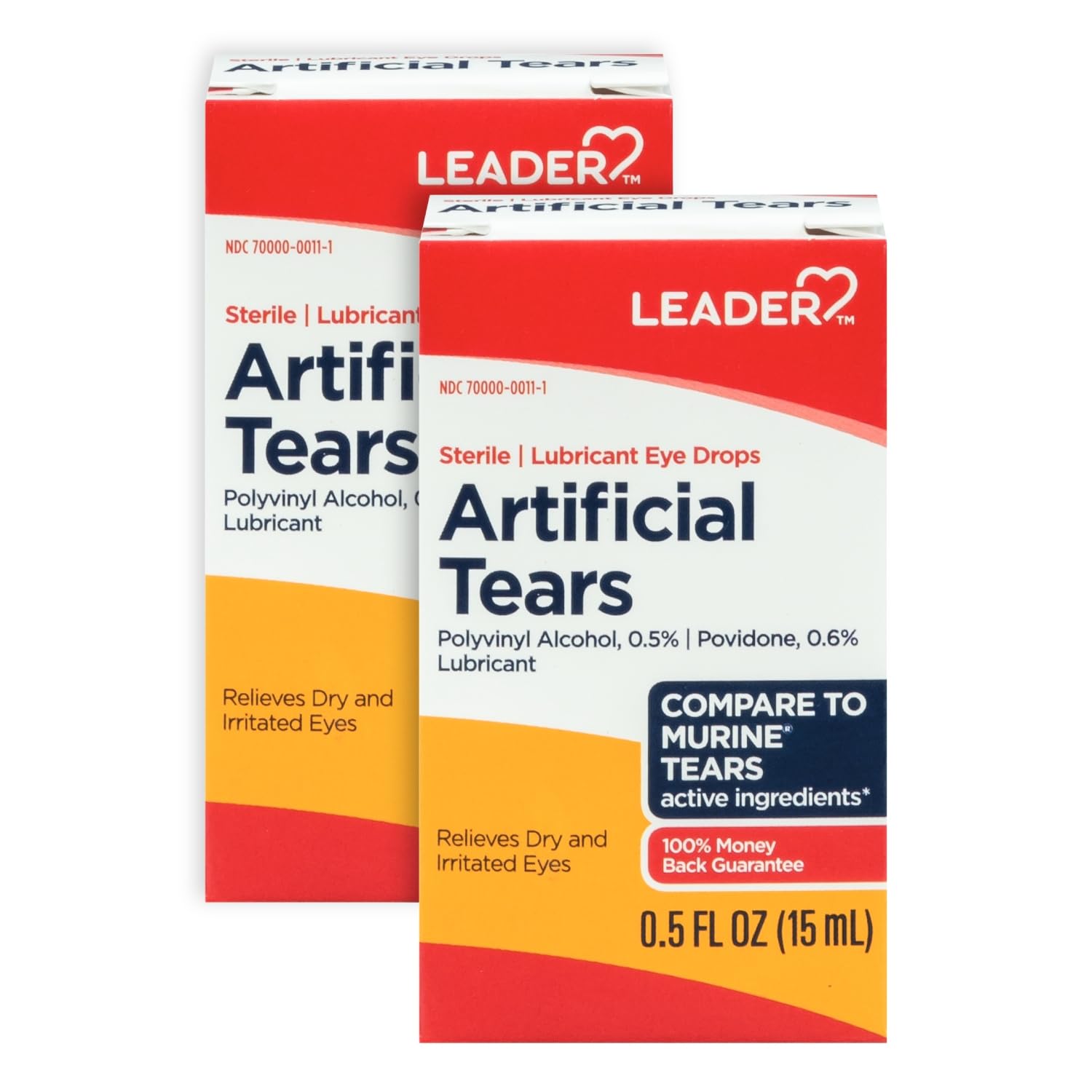
Leader Artificial Tears Recall 2025 Update. Earlier in 2023, ezricare artificial tears were recalled after reports of infections. Brs analytical service is voluntarily recalling over 75,000 cases of eye products, including various types of artificial tears,.

The recalls involve artificial tear eye drops, gels, and ointments. Over the course of 2023, 2024, and 2025, manufacturers have recalled various artificial tear products. The urgent recall of eye drops and artificial tears follows an fda audit.






![EzriCare Artificial Tears Lawsuit [April 2025 Update] Leader Artificial Tears Recall 2025 Update](https://www.torhoermanlaw.com/wp-content/uploads/2023/05/EzriCare-Artificial-Tears-Lawsuit.png)
![EzriCare Artificial Tears Lawsuit [April 2025 Update] Leader Artificial Tears Recall 2025 Update](https://www.torhoermanlaw.com/wp-content/uploads/2023/05/Gathering-Evidence-For-The-EzriCare-Recall-Lawsuit-2.webp)
![EzriCare Artificial Tears Lawsuit [April 2025 Update] Leader Artificial Tears Recall 2025 Update](https://www.torhoermanlaw.com/wp-content/uploads/2023/05/FDA-Warns-Consumers_-Possible-Contamination-Of-EzriCare-Artificial-Tears-And-Other-Eye-Drops.webp)
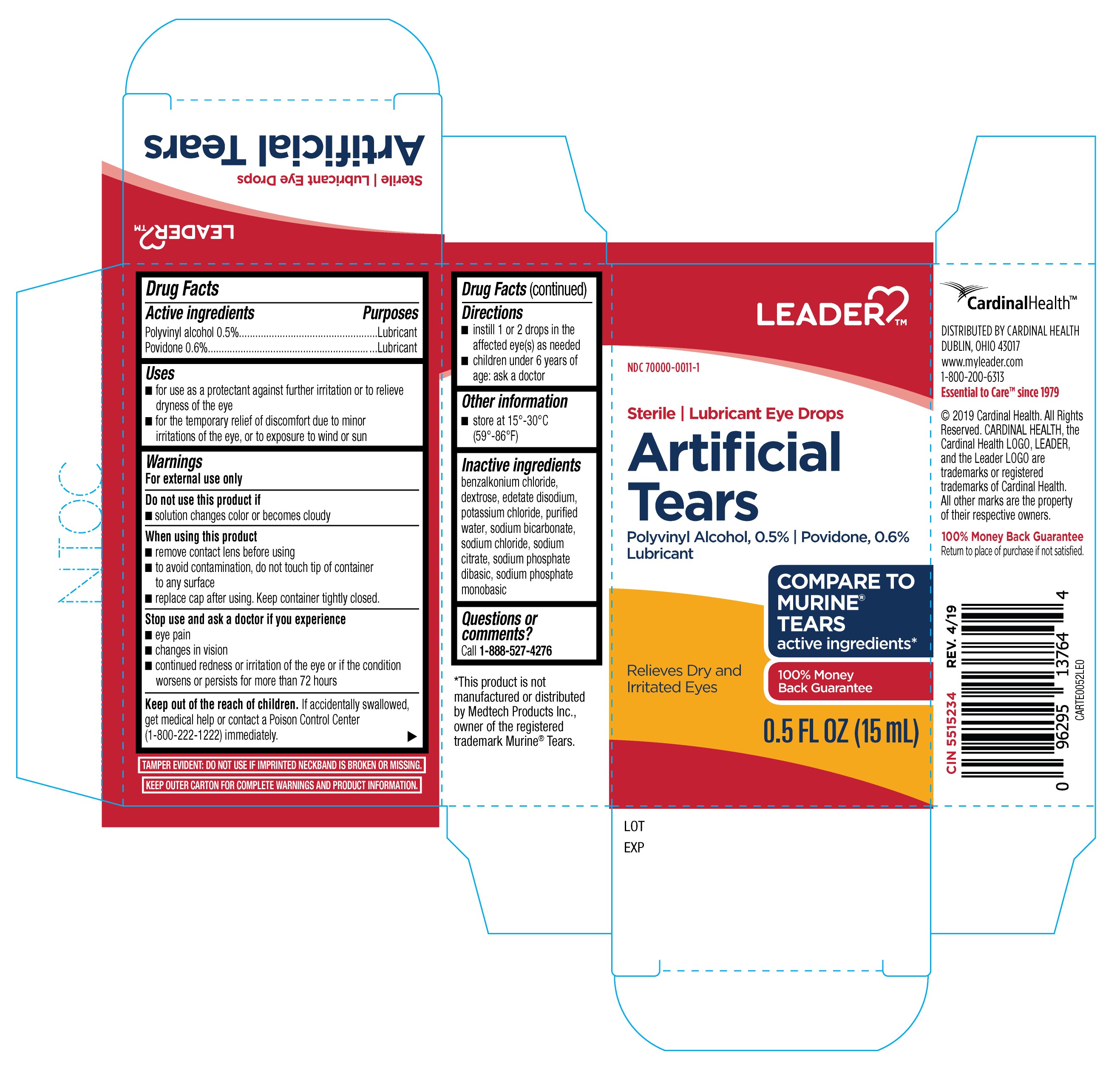

The Recalls Involve Artificial Tear Eye Drops, Gels, And Ointments.
These infections led to hospitalisations, vision. Earlier in 2023, ezricare artificial tears were recalled after reports of infections. The urgent recall of eye drops and artificial tears follows an fda audit.
Ophthalmic Products Were Voluntarily Recalled Due To A Manufacturing Deviation Discovered During An Audit By The U.s.
The recall involves the following products, according to the notice issued by avkare. Several eyedrop manufacturers have issued a recall of eyedrops and eyecare products in 2025 — here are the products that have been recalled. What eye drops are being recalled?
Over The Course Of 2023, 2024, And 2025, Manufacturers Have Recalled Various Artificial Tear Products.
The fda and avkare have reported a recall of eye care products, including drops and artificial tears, from brs analytical service. Brs analytical service is voluntarily recalling over 75,000 cases of eye products, including various types of artificial tears,.